learn about our research
Objective
our lab aims to model and control stem cell behaviors
including decisions related to cell type specification, communication between cells, and morphogenesis – or organization and patterning – of groups of cells in engineered tissues.
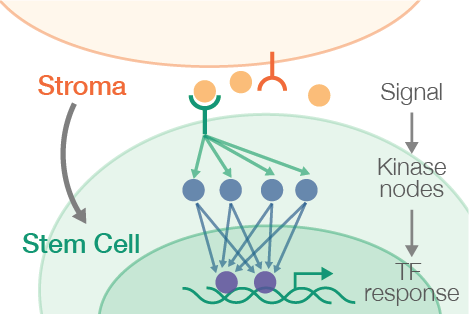
How do cells work together to make decisions?
Common protocols for stem cell differentiation use stroma, often referred to as “feeder” cells, upon which stem cells are cultured. These feeders are thought to communicate with stem cells and provide a hospitable environment. While effective, this “black box” approach leaves gaping holes in our understanding of the signals that mediate stem cell decisions. In addition, a more defined system – ideally replacing feeder cells with these few, critical signals – will ultimately be required to manufacture cells which meet regulatory standards for reproducibility and patient safety. Therefore, our primary goals are to: 1) outline the scope of cell-cell communication 2) understand the how stem cell responds to multiple simultaneous stimuli, and 3) develop methods to mimic the presence of feeders.
How do cells collectively organize?
Stem cell differentiation does not appear to be completely random. In cultures where the cells form aggregates, or spheroids, we do not necessarily see a speckled, salt-and-pepper distribution of cell types. Instead, we find similar cells grouped together and in patterns that often look surprisingly reminiscent of human tissue. We are intrigued by this notion that cells can work together to create their own environment, or niche. By further understanding this process of cellular self-organization, we envision opportunities to further guide or engineer even more complex and authentic model tissues. The ultimate goal is to create the blood niche – the bone marrow – in a dish.
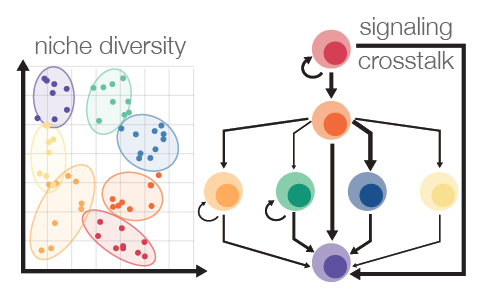
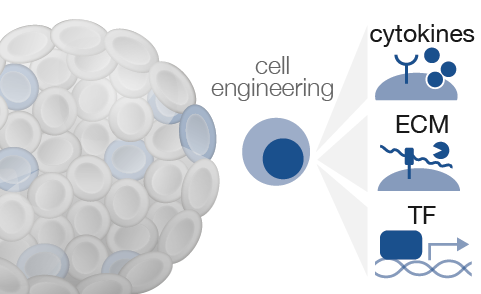
How do cells respond to local changes in their environment?
While cells can teach us a lot by observing how they naturally organize and differentiate, we can learn even more about a system by perturbing it. Advances in synthetic biology technologies, like CRISPR, allow us to be even more precise with these perturbations. Broadly, this project aims to understand how local perturbations affect differentiation and patterning. Understanding how cells respond will start to create a roadmap, somewhat akin to an electrical circuit diagram, for how to engineer cells and their environments.
Melissa A. Kinney, Ph.D.
Department Of Biomedical Engineering
University Of Wisconsin-Madison
Engineering Centers Building
Room 2148
1550 Engineering Dr
Madison, WI 53706

